The Laws of Adiposity: A Book Excerpt

What follows is a sample chapter from Gary Taubes’ newest book, Why We Get Fat: And What To Do About It.
The fate of the laboratory rat is rarely enviable. The story I’m about to tell offers no exception. Still, we can learn from the rat experience, as scientists do.
In the early 1970s, a young researcher at the University of Massachusetts named George Wade set out to study the relationship between sex hormones, weight and appetite by removing the ovaries from rats (females, obviously) and then monitoring their subsequent weight and behavior. The effects of the surgery were suitably dramatic: the rats would begin to eat voraciously and quickly became obese. If we didn’t know any better, we might assume from this that the removal of a rat’s ovaries makes it a glutton. The rat eats too much, the excess calories find their way to the fat tissue and the animal becomes obese. This would confirm our preconceptions about overeating being responsible for obesity in humans as well.
But Wade did a revealing second experiment, removing the ovaries from the rats and putting them on a strict post-surgical diet. Even if these rats were ravenously hungry after the surgery, even if they desperately wanted to be gluttons, they couldn’t satisfy their urge. In the lingo of experimental science, this second experiment controlled for overeating. The rats, post-surgery, were only allowed enough food to eat what they would have eaten had they never had the surgery.
What happened is not what you’d probably think. The rats got just as fat, just as quickly. But these rats were now completely sedentary. They moved only when movement was required to get food.
If we knew only about this second experiment, this, too, could confirm our preconceptions. Now we would assume that removing a rat’s ovaries makes it lazy; it expends too little energy, and this is why it gets fat. In this interpretation, once again we have confirmation of our belief in the primacy of calories-in-calories-out as the determining factor in obesity.
Pay attention to both experiments, though, and the conclusion is radically different. Removing the ovaries from a rat literally makes its fat tissue absorb calories from the circulation and expand with fat. If the animal can eat more to compensate for these calories that are now being stashed away as fat (the first experiment), it will. If it can’t (the second), then it expends less energy, because it now has less calories available to expend.
The animal doesn’t get fat because it overeats, as Wade said to me. It overeats because it’s getting fat. The cause and effect are reversed. Both gluttony and sloth are effects of the drive to get fatter. They are caused fundamentally by a defect in the regulation of the animal’s fat tissue. The removal of the ovaries literally makes the rat stockpile body fat; the animal either eats more or expends less energy, or both, to compensate.
To explain why this happens, I’m going to have to get technical for a moment. As it turns out, removing the rats’ ovaries serves the function of removing estrogen, the female sex hormone that is normally secreted by the ovaries. (When estrogen was infused back into the rats post-surgery, they neither ate voraciously, nor became slothful nor got obese. They acted like perfectly normal rats.) And one of the things that estrogen does in rats (and humans) is influence an enzyme called lipoprotein lipase -- LPL for short. What LPL does in turn, very simplistically, is pull fat from the bloodstream into whatever cell happens to “express” this LPL. If the LPL is attached to a fat cell, then it pulls fat from the circulation into the fat cell. The animal (or the person) in which that fat cell resides gets infinitesimally fatter. If the LPL is attached to a muscle cell, it pulls the fat into the muscle cell and the muscle cell burns it for fuel.
Estrogen happens to suppress or “inhibit” the activity of LPL on fat cells. The more estrogen around, the less LPL will be pulling fat out of the bloodstream and into the fat cells and the less fat those cells will accumulate. Get rid of the estrogen (by removing the ovaries) and fat cells blossom with LPL. The LPL then does what it always does – pulls fat into the cells -- but now the animal gets far fatter than normal, because now the fat cells have far more LPL doing that job.
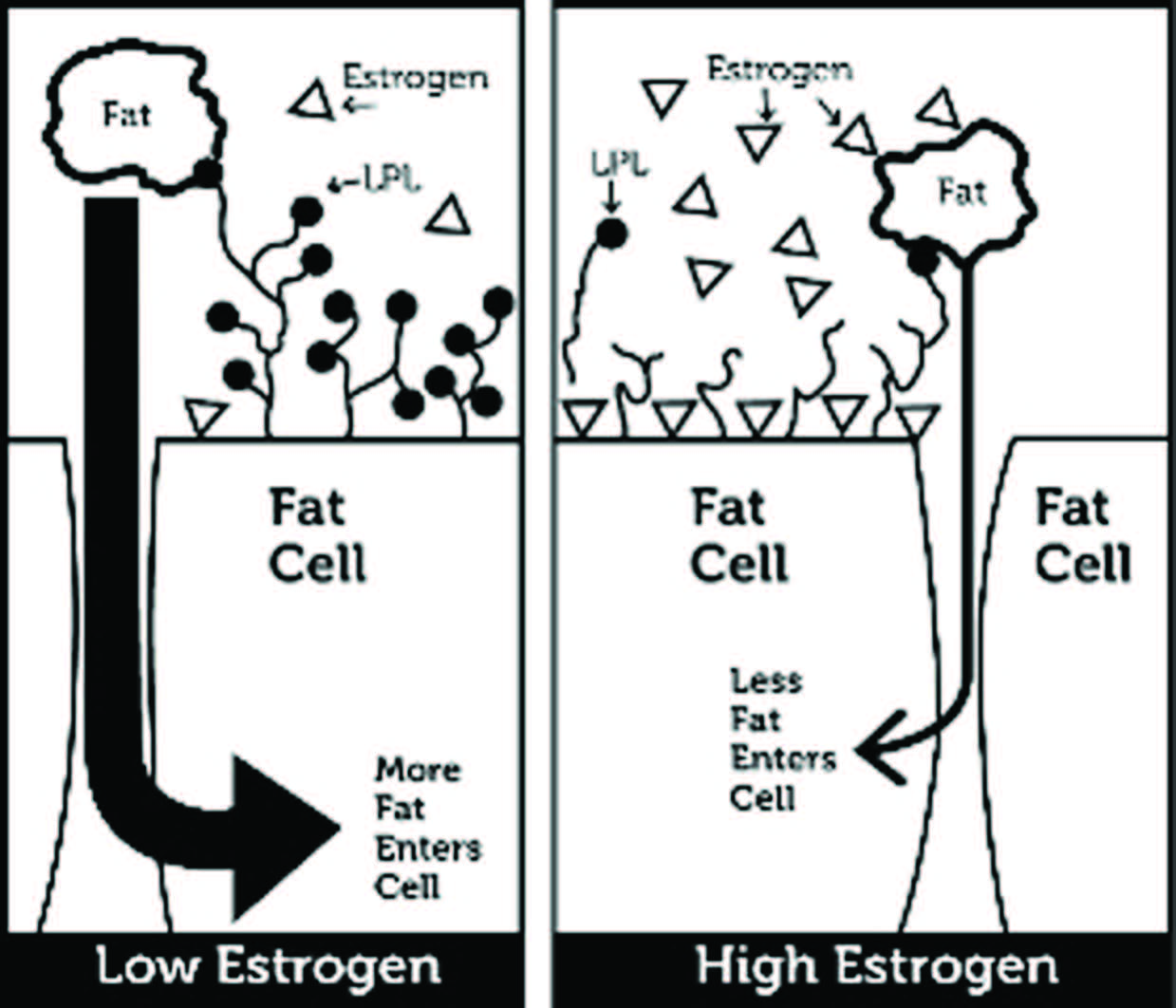
The animal has the urge to eat voraciously because it’s now losing calories into its fat cells that are needed elsewhere to run its body. The more calories its fat cells sequester, the more it must eat to compensate. The fat cells, in effect, are hogging calories, and there aren’t enough to go around for other cells. Now a meal that would previously have satisfied the animal no longer does. And because the animal is getting fatter (and heavier), this increases its caloric requirements even further. So the animal is ravenous, and if it can’t satisfy this newfound hunger, it has to settle for expending less energy.
The only way (short of more surgery) to stop these animals from getting fat – dieting has no effect and we can be confident that trying to force it to exercise would be futile – is to give them their estrogen back. When that is done, they become lean again and their appetite and energy levels return to normal.
So removing the ovaries from a rat literally makes its fat cells fatten. And this, very likely, is what happens to many women who get fat when they have their ovaries removed or after menopause. They secrete less estrogen, and their fat cells express more LPL.
The story of Wade and his rats reverses our perception of the cause and effect of obesity. It tells us that two behaviors – gluttony and sloth – that seem to be the reasons why we get fat can in fact be the effects of getting fat. It tells us that if we pay attention to the hormones and enzymes that regulate the fat tissue itself, we can understand precisely why this is so; not only why these rats get fat, but why they exhibit the behaviors that we typically associate with fat people.
Another remarkable aspect of the last half century of discussion about obesity and weight loss is that medical experts have been remarkably uninterested in the fat tissue itself and how our bodies happen to regulate it. With very few exceptions, they’ve simply ignored the fat tissue because they’ve already concluded that the problem is behavioral and lies in the brain, not in the body. Had we been discussing disorders of growth – why some people grow to be over seven feet tall and others never make it to four feet – the only subject of discussion would be the hormones and enzymes that regulate growth. And yet when we’re discussing a disorder in which the defining symptom is the abnormal growth of our fat tissue, the hormones and enzymes that regulate that growth are considered irrelevant.
When we pay attention to the regulation of our fat tissue, though, we arrive at an explanation for why we get fat and what to do about it that differs radically from the conventional thinking derived from the focus on the balance of energy consumed and expended. We have to conclude, as Wade did for his rats, that those who get fat do so because of the way their fat happens to be regulated and that a conspicuous consequence of this regulation is to cause the eating behavior (gluttony) and the physical inactivity (sloth) that we so readily assume are the actual causes.
I’m going to discuss this idea first as a hypothesis, a way of thinking about why we get fat that could be correct, and then I’m going to explain why it almost assuredly is.** Before I get to that, though, there are several critical points about fat and the process of fattening itself that you’ll have to understand. In honor of the laws of thermodynamics that they’re replacing, we’ll call these the laws of adiposity.
The First Law
Body fat is carefully regulated, if not exquisitely so.
This is true despite the fact that some people fatten so easily that it’s virtually impossible to imagine. What I mean by “regulated” is that our bodies, when healthy, are working diligently to maintain a set amount of fat in our fat tissue – not too much and not to little. The implication (our working assumption) is that if someone gets obese, it’s because this regulation has been thrown out of whack, not that it has ceased to exist.
The evidence that fat tissue is carefully regulated, not just a garbage can where we dump whatever calories we don’t burn, is incontrovertible. We can start with all the observations mentioned previously about the wheres, whens and whos of fattening. That men and women fatten differently tells us that sex hormones play a role in regulating body fat (as do Wade’s experiment and what we know about estrogen and LPL). That some parts of our bodies are relatively fat free – the backs of our hands, for example, and our foreheads – and others not so tells us that local factors play a role in where we fatten - just as local factors obviously play a role in where we grow hair – in some places, but not in others.
That obesity runs in families (we’re more likely to be fat if our parents were fat) and that the local distribution of fat itself can be a genetic attribute (the steatopygia of certain African tribes) tells us that body fat is regulated, because how else would the genes passed from generation to generation influence our fat and where we put it, if not through the hormones and enzymes and other factors that regulate it.
That animals carefully regulate the amount of fat (and even the type of fat) they carry also argues for this conclusion. We are, after all, just another species of animal. Animals in the wild may be naturally fat (hippopotami, for instance, and whales). They’ll put on fat seasonally, as insulation in preparation for the cold of winter or as fuel for annual migrations or hibernations. Females will fatten in preparation for giving birth; males will fatten to give them a weight advantage in fights for females. But they never get obese, meaning they won’t suffer adverse health consequences from the fat the way humans do. They won’t become diabetic, for instance.
No matter how abundant their food supply, wild animals will maintain a stable weight – not too fat, not too thin -- which tells us that their bodies are assuring that the amount of fat in their fat tissue always works to their advantage and never becomes a hindrance to survival. When animals do put on significant fat, that fat is always there for a very good reason. The animal will be as healthy with it as without.
Excellent examples of how carefully animals (and so presumably humans, too) regulate their fat accumulation are hibernating rodents -- ground squirrels, for example, which double their weight and body fat in just a few weeks of late summer. Dissecting these squirrels at their peak weight, as one researcher described it to me, is like “opening a can of Crisco oil -- enormous gobs of fat, all over the place.”
But these squirrels will accumulate this fat regardless of how much they eat, just like Wade’s ovary-less rats. They can be housed in a laboratory and kept to a strict diet from springtime, when they awake from hibernation, through late summer and they’ll get just as fat as squirrels allowed to eat to their heart’s content. They’ll burn the fat through the winter and lose it at the same rate, whether they remain awake in a warm laboratory with food available, or go into full hibernation, eating not a bite and surviving solely off their fat supplies.
The fact is there’s very few things that researchers can do to keep these animals from gaining and losing fat on schedule. Manipulating the food available, short of starving them to death, is not one of them. The amount of fat on these rodents at any particular time of the year is regulated entirely by biological factors, not the food available or the amount of energy required to get that food. And this makes perfect sense. If an animal that requires enormous gobs of fat for its winter fuel supply were to require excessive amounts of food to accumulate that fat, then one bad summer would have long ago wiped out the entire species.
It may be true that evolution has singled out humans as the sole species on the planet that does not work to carefully regulate its fat stores in response to periods of both feast and famine, that some people will stockpile so much fat merely because food is available in abundance that they become virtually immobile; but it requires that we ignore virtually everything we know about evolution to accept this conclusion.
A final argument for the careful regulation of body fat is the fact that everything else in our bodies is meticulously regulated. Why would fat be an exception? When regulation breaks down, as it does in cancer and heart disease, the result is often fatally obvious. When people accumulate excess fat, it tells us that something has gone awry in the careful regulation of their fat tissue. What we need to know is what that defect is and what to do about it.
The Second Law
Obesity can be caused by a regulatory defect so small that it would be undetectable by any technique yet invented.
Remember the twenty-calorie-a-day problem we discussed earlier? If we overeat by just twenty calories each day – one percent or less than our typical daily caloric quota – that’s enough to transform us from lean in our twenties to obese in our fifties. In the context of the calories-in-calories-out logic, this led to the obvious question: How does anyone remain lean if it requires that we consciously balance the calories we eat to those we expend with an accuracy of better than one percent? That seems impossible and assuredly is.
Well, these same twenty calories a day are all this regulatory system has to misdirect into our fat cells to make us obese. The same arithmetic applies. If, by some unlucky combination of genes and environment, a regulatory error causes our fat cells to store in excess just one percent of the calories that would otherwise be used for fuel, then we are destined to become obese and there’s precious little we can do about it. (Luckily, as you’ll see, there is.)
This misappropriation of calories into fat need be only slightly larger and someone could end up grotesquely fat. Yet this would still seem like a relatively minor error in regulatory judgment – just a few percent, something exceedingly difficult to measure and yet not that hard to imagine.
The Third Law
Whatever makes us both fatter and heavier will also make us overeat.
This was the ultimate lesson of Wade’s rats. It may be counterintuitive, but it has to be true for every species, for every person who puts on pounds of fat. Its arguably the one lesson you (and our health experts) have to learn to understand why we get fat and what to do about it.
This law is one fact we can count on from the first law of thermodynamics, the law of energy conservation that health experts have been so determined to misapply. Anything that increases its mass, for whatever reason, will take in more energy than it expends. So if a regulatory defect makes us both fatter and heavier, it is guaranteed to make us consume more calories and so increase our appetite and/or expend less than would be the case if this regulation was working perfectly.
Here’s where growing children help as a metaphor to understand this cause-and-effect of getting fat and overeating. I’m going to use two photos of my oldest son to make this point. Here’s one taken when he was not quite two years old and weighed thirty-four pounds.

Here’s one taken three years later, after he gained nine inches in height and weighed fifty-one pounds.

He gained seventeen pounds in three years, so he certainly consumed more calories than he expended. He overate. Those excess calories were used to create all the necessary tissues and structures that a larger body needed, including, yes, even more fat. But he didn’t grow because he consumed excess calories. He consumed those excess calories -- he overate -- because he was growing.
My son’s growth, as with every child’s, is caused fundamentally by the action of growth hormones. As he gets older, he’ll occasionally go through growth spurts that will be accompanied by a voracious appetite and probably a fair share of sloth, but the appetite and the sloth will be driven by the growth, not vice versa. His body will require excess calories to satisfy the demands of the growth – to build a bigger body -- and it will figure out a way to get them, through increasing his appetite or decreasing his energy expenditure or both. When he goes through puberty, he’ll lose fat and gain muscle; he’ll still be taking in more calories than he expends, and this, too, will be driven by hormonal changes.
That growth is the cause and overeating the effect is almost assuredly true for our fat tissue as well. To paraphrase what the German internist Gustav von Bergmann said about this idea eighty years ago, we would never even consider the possibility that children grow taller because they eat too much and exercise too little (or that they stunt their growth by exercising too much). So why assume that these are valid explanations for growing fat (or remaining lean)? “That which the body needs to grow it always finds,” von Bergmann wrote, “and that which it needs to become fat, even if it’s ten times as much, the body will save for itself from the annual balance.”
The only reason to think this isn’t true, that the cause and effect go in one direction when we get taller (growth causes overeating) and the other when we grow fatter (overeating causes growth) is because this is what we grew up believing and we never stopped to consider if it actually made sense. The far more reasonable assumption is that growth in both cases determines appetite and even energy expenditure, not the other way around. We don’t get fat because we overeat; we overeat because we’re getting fat.
Since this is so counter-intuitive but so critical to understand, I want to return to the examples of animals. African elephants are the world’s largest land animals. The males typically weigh more than 10,000 pounds, although surprisingly little of this is fat. Blue whales are the largest animals, on or off land. They can weigh more than 300,000 pounds and much of that is fat. African elephants will eat hundreds of pounds of food a day, and blue whales, thousands, prodigious amounts, but neither grow to be enormous because they so eat so much. They eat prodigious amounts, because they’re enormous animals. With or without large quantities of body fat, body size determines how much they eat.
The infants of these species also eat relatively enormous quantities. They do so because they’re born exceedingly large to begin with and because their genes predispose them to grow many thousand pounds (elephants) or hundreds of thousands of pounds (blue whales) larger still. Now both growth and body size are driving appetite. This is true whether these animals are using the calories to store fat or enlarge muscle and other tissues and organs. Whether or not they have enormous quantities of fat, the same cause and effect holds true.
Now consider what researchers call animal models of obesity – animals, like Wade’s rats, that are made obese in the laboratory, but wouldn’t be naturally. Over the past eighty years, researchers have learned that they can make rats and mice obese by breeding, by surgery (removing the ovaries, for instance), by manipulating their diets and by any number of genetic manipulations. The animals on which these indignities are inflicted do indeed become obese, not just functionally fat (like blue whales or hibernating ground squirrels). They tend to suffer from the same metabolic disturbances, including diabetes, that we do when we become obese.
It doesn’t matter, though, what technique is used to make the animals obese, they’ll still get that way or at least significantly fatter (just as Wade’s rats did) whether or not they can eat any more calories than otherwise identical animals that remain lean. They get obese not because they overeat, but because the surgery or breeding or genetic manipulation or even the change in diet disturbed the regulation of their fat tissue. They began stockpiling calories as fat, and then their bodies had to compensate: they ate more, if possible; they expended less energy if not. Often they do both.
Take, for example, the preferred method of making obese laboratory rodents from the 1930s through the 1970s. This was a surgical technique that required inserting a needle into a part of the brain known as the hypothalamus, which controls (not coincidentally) hormone secretion throughout the body. After the surgery, some of these rodents would eat voraciously and get obese; some would become sedentary and get obese; some would do both and get obese. The obvious conclusion, suggested first by the neuroanatomist Stephen Ranson (whose Northwestern University laboratory pioneered these experiments in the 1930s), is that the surgery has the direct effect of increasing body fat on these rodents. After the surgery, their fat tissue sucks up calories to make more fat; this leaves insufficient fuel for the rest of the body – what Ranson called “hidden semi-cellular starvation” – and “force[s] the body either to increase its general food intake or to cut down its expenditure, or both.”
The only way to prevent these animals from getting obese is to starve them – to inflict what a Johns Hopkins University physiologist in the 1940s called “severe and permanent” food restriction. If these animals are allowed to eat even moderate amounts of food, they end up obese. In other words, they get fat not by overeating, but by eating at all. Despite the fact that the surgery is in the brain, it has the effect of fundamentally altering the regulation of body fat, not appetite.
The same thing holds true for animals that are bred to be obese, for which obesity is in their genes. In the 1950s, Jean Mayer studied one such strain of obese mice in his Harvard laboratory. As he reported it, he could get their weights below that of lean mice if he starved them sufficiently, but they’d “still contain more fat than the normal ones, while their muscles have melted away.” Once again, eating too much wasn’t the problem; these mice, as Mayer wrote, “will make fat out of their food under the most unlikely circumstances, even when half starved.”
Then there are Zucker rats, which researchers began studying in the 1950s and are still a favorite obesity model today. Here’s a picture of a Zucker rat looking suitably corpulent:

These rats, like Mayer’s mice, are genetically predisposed to get fat. When these Zucker rats are put on a calorie-restricted diet from the moment they’re weaned from their mother’s milk, they don’t end up leaner than their littermates who are allowed to eat as much as they want. They end up fatter. They may weigh a little less, but they have just as much or even more body fat. Even if they want to be gluttons, which they assuredly do, they can’t, and still they get even fatter than they would have had they never been put on a diet. On the other hand, their muscles and organs, including their brains and kidneys, are smaller than they’d otherwise be. Just as the muscles in Mayer’s mice “melted away” when starved, the muscles and organs in these semi-starved Zucker rats are “significantly reduced” in size compared to those fat littermates who get to eat freely. “In order to develop this obese body composition in the face of calorie restriction,” wrote the researcher who reported this observation in 1981, “several developing organ systems in the obese rats [are] compromised.”
Let’s think about this for a second. If a baby rat that is genetically programmed to become obese is put it on a diet from the moment it’s weaned, so it can never eat as much as it would like – indeed, no more than a lean rat would eat -- it responds by compromising its organs and muscles to satisfy its genetic drive to grow fat. It’s not just using the energy it would normally expend in day-to-day activity to grow fat, it’s taking the materials and the energy it would normally dedicate to building its muscles, organs and even its brain and using that.
When these obese rodents are starved to death—an experiment that thankfully not too many researchers have done—a common result reported in the literature is that the animals die with much of their fat tissue intact. In fact, they’ll often die with more body fat than lean animals have when the lean ones are eating as much as they like. As animals starve, and the same is true of humans, they consume their muscles for fuel and that includes, eventually, their heart muscle. So as adults, these obese animals are also willing to compromise their organs and even their heart and their life to preserve their fat.
The message of eighty years of research on obese animals is simple and unconditional and worth restating: obesity does not come about because gluttony and sloth makes it so. Changing the regulation of the fat tissue makes lean animals obese, nothing else.
The amount of body fat on these animals is determined by a balance of all the various forces that work on the fat tissue – on the fat cells, as we’ll see -- to either put fat in or get fat out. Whatever’s been done to these animals to make them fat (surgery, genetic manipulation, etc.), the effect is literally to change this balance of forces so that the animals increase their fat stores. Now “eating too much” is a meaningless concept because virtually any amount of food is “too much.” The fat tissue is not reacting to how much these animals are eating but only to the forces making it accumulate fat. And because increasing body fat requires energy and nutrients that would otherwise be needed elsewhere in their bodies, they will eat more if they can. If they can’t – if they are on a strict diet – they will expend less energy because they have less to expend. They may even compromise their brains, muscles and other organs. Half-starve these animals and they’ll still find a way to stockpile calories as fat because that’s what their fat tissue is now programmed to do.
If this is true of humans, and there’s little reason to think it’s not, this is the explanation for the paradigm-challenging observation I mentioned earlier regarding extremely poor but overweight mothers with thin, stunted children. Both mother and children are half-starved. The children, thin and their growth stunted, respond as we’d expect. The mothers, however, have fat tissue that has developed its own agenda. (We’ll see shortly how this can happen.) It will accumulate excess fat, and does so, despite the fact that the mothers themselves, like their children, are barely getting enough food to survive. They must be expending less energy to compensate.
Before I leave the laws of adiposity and this animal research behind, I want to ask one more question: What do these laws and this research have to say about people who are habitually lean? Over the years, researchers have also created what we might call animal models of leanness – animals whose genes have been manipulated so they are leaner than they’d otherwise be. These animals will remain lean even when the researchers force them to consume more calories than they prefer – by infusing a tube into their guts, for instance, and pumping in calories directly. In such cases, the animals will surely have to increase their expenditure to burn off the calories.
The implication is as counterintuitive as anything we’ve discussed so far. Just as the animal research tells us that gluttony and sloth are side effects of a drive to accumulate body fat, it also says that eating in moderation and being physically active (literally, having the energy to exercise) are not evidence of moral rectitude. Rather they’re the metabolic benefits of a body that’s programmed to remain lean. If our fat tissue is regulated so that it will not store significant calories as fat, or our muscle tissue is regulated to take up more than its fair share of calories to use for fuel, then we’ll either eat less than those of us predisposed to be fat (the first case), or we’ll be more physically active (the second), or both because of it.
This implies that our emaciated marathoners are not lean because they train religiously and burn off thousands of calories doing so; rather they’re driven to expend those calories – and so perhaps to work out for hours a day and become obsessive long-distance runners -- because they’re wired to burn off calories and be lean. A greyhound will similarly be more physically active than a basset hound, not because of any conscious desire to exercise, but because its body partitions fuel to its lean tissue, rather than its fat.
It maybe easier to believe that we remain lean because we’re virtuous and we get fat because we’re not, but the evidence simply says otherwise. Virtue almost assuredly has no more to do with our weight than with our height. When we grow taller, it’s hormones and enzymes that spur our growth and we consume more calories than we expend as a result. Growth is the cause – increased appetite and decreased energy expenditure (gluttony and sloth) are the effects. When we grow fatter, the same is true as well.
We don’t get fat because we overeat; we overeat because we’re getting fat.
The fate of the laboratory rat is rarely enviable. The story I’m about to tell offers no exception. Still, we can learn from the rat experience, as scientists do.
In the early 1970s, a young researcher at the University of Massachusetts named George Wade set out to study the relationship between sex hormones, weight and appetite by removing the ovaries from rats (females, obviously) and then monitoring their subsequent weight and behavior. The effects of the surgery were suitably dramatic: the rats would begin to eat voraciously and quickly became obese. If we didn’t know any better, we might assume from this that the removal of a rat’s ovaries makes it a glutton. The rat eats too much, the excess calories find their way to the fat tissue and the animal becomes obese. This would confirm our preconceptions about overeating being responsible for obesity in humans as well.
But Wade did a revealing second experiment, removing the ovaries from the rats and putting them on a strict post-surgical diet. Even if these rats were ravenously hungry after the surgery, even if they desperately wanted to be gluttons, they couldn’t satisfy their urge. In the lingo of experimental science, this second experiment controlled for overeating. The rats, post-surgery, were only allowed enough food to eat what they would have eaten had they never had the surgery.
What happened is not what you’d probably think. The rats got just as fat, just as quickly. But these rats were now completely sedentary. They moved only when movement was required to get food.
If we knew only about this second experiment, this, too, could confirm our preconceptions. Now we would assume that removing a rat’s ovaries makes it lazy; it expends too little energy, and this is why it gets fat. In this interpretation, once again we have confirmation of our belief in the primacy of calories-in-calories-out as the determining factor in obesity.
Pay attention to both experiments, though, and the conclusion is radically different. Removing the ovaries from a rat literally makes its fat tissue absorb calories from the circulation and expand with fat. If the animal can eat more to compensate for these calories that are now being stashed away as fat (the first experiment), it will. If it can’t (the second), then it expends less energy, because it now has less calories available to expend.
The animal doesn’t get fat because it overeats, as Wade said to me. It overeats because it’s getting fat. The cause and effect are reversed. Both gluttony and sloth are effects of the drive to get fatter. They are caused fundamentally by a defect in the regulation of the animal’s fat tissue. The removal of the ovaries literally makes the rat stockpile body fat; the animal either eats more or expends less energy, or both, to compensate.
To explain why this happens, I’m going to have to get technical for a moment. As it turns out, removing the rats’ ovaries serves the function of removing estrogen, the female sex hormone that is normally secreted by the ovaries. (When estrogen was infused back into the rats post-surgery, they neither ate voraciously, nor became slothful nor got obese. They acted like perfectly normal rats.) And one of the things that estrogen does in rats (and humans) is influence an enzyme called lipoprotein lipase -- LPL for short. What LPL does in turn, very simplistically, is pull fat from the bloodstream into whatever cell happens to “express” this LPL. If the LPL is attached to a fat cell, then it pulls fat from the circulation into the fat cell. The animal (or the person) in which that fat cell resides gets infinitesimally fatter. If the LPL is attached to a muscle cell, it pulls the fat into the muscle cell and the muscle cell burns it for fuel.
Estrogen happens to suppress or “inhibit” the activity of LPL on fat cells. The more estrogen around, the less LPL will be pulling fat out of the bloodstream and into the fat cells and the less fat those cells will accumulate. Get rid of the estrogen (by removing the ovaries) and fat cells blossom with LPL. The LPL then does what it always does – pulls fat into the cells -- but now the animal gets far fatter than normal, because now the fat cells have far more LPL doing that job.

The animal has the urge to eat voraciously because it’s now losing calories into its fat cells that are needed elsewhere to run its body. The more calories its fat cells sequester, the more it must eat to compensate. The fat cells, in effect, are hogging calories, and there aren’t enough to go around for other cells. Now a meal that would previously have satisfied the animal no longer does. And because the animal is getting fatter (and heavier), this increases its caloric requirements even further. So the animal is ravenous, and if it can’t satisfy this newfound hunger, it has to settle for expending less energy.
The only way (short of more surgery) to stop these animals from getting fat – dieting has no effect and we can be confident that trying to force it to exercise would be futile – is to give them their estrogen back. When that is done, they become lean again and their appetite and energy levels return to normal.
So removing the ovaries from a rat literally makes its fat cells fatten. And this, very likely, is what happens to many women who get fat when they have their ovaries removed or after menopause. They secrete less estrogen, and their fat cells express more LPL.
The story of Wade and his rats reverses our perception of the cause and effect of obesity. It tells us that two behaviors – gluttony and sloth – that seem to be the reasons why we get fat can in fact be the effects of getting fat. It tells us that if we pay attention to the hormones and enzymes that regulate the fat tissue itself, we can understand precisely why this is so; not only why these rats get fat, but why they exhibit the behaviors that we typically associate with fat people.
Another remarkable aspect of the last half century of discussion about obesity and weight loss is that medical experts have been remarkably uninterested in the fat tissue itself and how our bodies happen to regulate it. With very few exceptions, they’ve simply ignored the fat tissue because they’ve already concluded that the problem is behavioral and lies in the brain, not in the body. Had we been discussing disorders of growth – why some people grow to be over seven feet tall and others never make it to four feet – the only subject of discussion would be the hormones and enzymes that regulate growth. And yet when we’re discussing a disorder in which the defining symptom is the abnormal growth of our fat tissue, the hormones and enzymes that regulate that growth are considered irrelevant.
When we pay attention to the regulation of our fat tissue, though, we arrive at an explanation for why we get fat and what to do about it that differs radically from the conventional thinking derived from the focus on the balance of energy consumed and expended. We have to conclude, as Wade did for his rats, that those who get fat do so because of the way their fat happens to be regulated and that a conspicuous consequence of this regulation is to cause the eating behavior (gluttony) and the physical inactivity (sloth) that we so readily assume are the actual causes.
I’m going to discuss this idea first as a hypothesis, a way of thinking about why we get fat that could be correct, and then I’m going to explain why it almost assuredly is.** Before I get to that, though, there are several critical points about fat and the process of fattening itself that you’ll have to understand. In honor of the laws of thermodynamics that they’re replacing, we’ll call these the laws of adiposity.
The First Law
Body fat is carefully regulated, if not exquisitely so.
This is true despite the fact that some people fatten so easily that it’s virtually impossible to imagine. What I mean by “regulated” is that our bodies, when healthy, are working diligently to maintain a set amount of fat in our fat tissue – not too much and not to little. The implication (our working assumption) is that if someone gets obese, it’s because this regulation has been thrown out of whack, not that it has ceased to exist.
The evidence that fat tissue is carefully regulated, not just a garbage can where we dump whatever calories we don’t burn, is incontrovertible. We can start with all the observations mentioned previously about the wheres, whens and whos of fattening. That men and women fatten differently tells us that sex hormones play a role in regulating body fat (as do Wade’s experiment and what we know about estrogen and LPL). That some parts of our bodies are relatively fat free – the backs of our hands, for example, and our foreheads – and others not so tells us that local factors play a role in where we fatten - just as local factors obviously play a role in where we grow hair – in some places, but not in others.
That obesity runs in families (we’re more likely to be fat if our parents were fat) and that the local distribution of fat itself can be a genetic attribute (the steatopygia of certain African tribes) tells us that body fat is regulated, because how else would the genes passed from generation to generation influence our fat and where we put it, if not through the hormones and enzymes and other factors that regulate it.
That animals carefully regulate the amount of fat (and even the type of fat) they carry also argues for this conclusion. We are, after all, just another species of animal. Animals in the wild may be naturally fat (hippopotami, for instance, and whales). They’ll put on fat seasonally, as insulation in preparation for the cold of winter or as fuel for annual migrations or hibernations. Females will fatten in preparation for giving birth; males will fatten to give them a weight advantage in fights for females. But they never get obese, meaning they won’t suffer adverse health consequences from the fat the way humans do. They won’t become diabetic, for instance.
No matter how abundant their food supply, wild animals will maintain a stable weight – not too fat, not too thin -- which tells us that their bodies are assuring that the amount of fat in their fat tissue always works to their advantage and never becomes a hindrance to survival. When animals do put on significant fat, that fat is always there for a very good reason. The animal will be as healthy with it as without.
Excellent examples of how carefully animals (and so presumably humans, too) regulate their fat accumulation are hibernating rodents -- ground squirrels, for example, which double their weight and body fat in just a few weeks of late summer. Dissecting these squirrels at their peak weight, as one researcher described it to me, is like “opening a can of Crisco oil -- enormous gobs of fat, all over the place.”
But these squirrels will accumulate this fat regardless of how much they eat, just like Wade’s ovary-less rats. They can be housed in a laboratory and kept to a strict diet from springtime, when they awake from hibernation, through late summer and they’ll get just as fat as squirrels allowed to eat to their heart’s content. They’ll burn the fat through the winter and lose it at the same rate, whether they remain awake in a warm laboratory with food available, or go into full hibernation, eating not a bite and surviving solely off their fat supplies.
The fact is there’s very few things that researchers can do to keep these animals from gaining and losing fat on schedule. Manipulating the food available, short of starving them to death, is not one of them. The amount of fat on these rodents at any particular time of the year is regulated entirely by biological factors, not the food available or the amount of energy required to get that food. And this makes perfect sense. If an animal that requires enormous gobs of fat for its winter fuel supply were to require excessive amounts of food to accumulate that fat, then one bad summer would have long ago wiped out the entire species.
It may be true that evolution has singled out humans as the sole species on the planet that does not work to carefully regulate its fat stores in response to periods of both feast and famine, that some people will stockpile so much fat merely because food is available in abundance that they become virtually immobile; but it requires that we ignore virtually everything we know about evolution to accept this conclusion.
A final argument for the careful regulation of body fat is the fact that everything else in our bodies is meticulously regulated. Why would fat be an exception? When regulation breaks down, as it does in cancer and heart disease, the result is often fatally obvious. When people accumulate excess fat, it tells us that something has gone awry in the careful regulation of their fat tissue. What we need to know is what that defect is and what to do about it.
The Second Law
Obesity can be caused by a regulatory defect so small that it would be undetectable by any technique yet invented.
Remember the twenty-calorie-a-day problem we discussed earlier? If we overeat by just twenty calories each day – one percent or less than our typical daily caloric quota – that’s enough to transform us from lean in our twenties to obese in our fifties. In the context of the calories-in-calories-out logic, this led to the obvious question: How does anyone remain lean if it requires that we consciously balance the calories we eat to those we expend with an accuracy of better than one percent? That seems impossible and assuredly is.
Well, these same twenty calories a day are all this regulatory system has to misdirect into our fat cells to make us obese. The same arithmetic applies. If, by some unlucky combination of genes and environment, a regulatory error causes our fat cells to store in excess just one percent of the calories that would otherwise be used for fuel, then we are destined to become obese and there’s precious little we can do about it. (Luckily, as you’ll see, there is.)
This misappropriation of calories into fat need be only slightly larger and someone could end up grotesquely fat. Yet this would still seem like a relatively minor error in regulatory judgment – just a few percent, something exceedingly difficult to measure and yet not that hard to imagine.
The Third Law
Whatever makes us both fatter and heavier will also make us overeat.
This was the ultimate lesson of Wade’s rats. It may be counterintuitive, but it has to be true for every species, for every person who puts on pounds of fat. Its arguably the one lesson you (and our health experts) have to learn to understand why we get fat and what to do about it.
This law is one fact we can count on from the first law of thermodynamics, the law of energy conservation that health experts have been so determined to misapply. Anything that increases its mass, for whatever reason, will take in more energy than it expends. So if a regulatory defect makes us both fatter and heavier, it is guaranteed to make us consume more calories and so increase our appetite and/or expend less than would be the case if this regulation was working perfectly.
Here’s where growing children help as a metaphor to understand this cause-and-effect of getting fat and overeating. I’m going to use two photos of my oldest son to make this point. Here’s one taken when he was not quite two years old and weighed thirty-four pounds.

Here’s one taken three years later, after he gained nine inches in height and weighed fifty-one pounds.

He gained seventeen pounds in three years, so he certainly consumed more calories than he expended. He overate. Those excess calories were used to create all the necessary tissues and structures that a larger body needed, including, yes, even more fat. But he didn’t grow because he consumed excess calories. He consumed those excess calories -- he overate -- because he was growing.
My son’s growth, as with every child’s, is caused fundamentally by the action of growth hormones. As he gets older, he’ll occasionally go through growth spurts that will be accompanied by a voracious appetite and probably a fair share of sloth, but the appetite and the sloth will be driven by the growth, not vice versa. His body will require excess calories to satisfy the demands of the growth – to build a bigger body -- and it will figure out a way to get them, through increasing his appetite or decreasing his energy expenditure or both. When he goes through puberty, he’ll lose fat and gain muscle; he’ll still be taking in more calories than he expends, and this, too, will be driven by hormonal changes.
That growth is the cause and overeating the effect is almost assuredly true for our fat tissue as well. To paraphrase what the German internist Gustav von Bergmann said about this idea eighty years ago, we would never even consider the possibility that children grow taller because they eat too much and exercise too little (or that they stunt their growth by exercising too much). So why assume that these are valid explanations for growing fat (or remaining lean)? “That which the body needs to grow it always finds,” von Bergmann wrote, “and that which it needs to become fat, even if it’s ten times as much, the body will save for itself from the annual balance.”
The only reason to think this isn’t true, that the cause and effect go in one direction when we get taller (growth causes overeating) and the other when we grow fatter (overeating causes growth) is because this is what we grew up believing and we never stopped to consider if it actually made sense. The far more reasonable assumption is that growth in both cases determines appetite and even energy expenditure, not the other way around. We don’t get fat because we overeat; we overeat because we’re getting fat.
Since this is so counter-intuitive but so critical to understand, I want to return to the examples of animals. African elephants are the world’s largest land animals. The males typically weigh more than 10,000 pounds, although surprisingly little of this is fat. Blue whales are the largest animals, on or off land. They can weigh more than 300,000 pounds and much of that is fat. African elephants will eat hundreds of pounds of food a day, and blue whales, thousands, prodigious amounts, but neither grow to be enormous because they so eat so much. They eat prodigious amounts, because they’re enormous animals. With or without large quantities of body fat, body size determines how much they eat.
The infants of these species also eat relatively enormous quantities. They do so because they’re born exceedingly large to begin with and because their genes predispose them to grow many thousand pounds (elephants) or hundreds of thousands of pounds (blue whales) larger still. Now both growth and body size are driving appetite. This is true whether these animals are using the calories to store fat or enlarge muscle and other tissues and organs. Whether or not they have enormous quantities of fat, the same cause and effect holds true.
Now consider what researchers call animal models of obesity – animals, like Wade’s rats, that are made obese in the laboratory, but wouldn’t be naturally. Over the past eighty years, researchers have learned that they can make rats and mice obese by breeding, by surgery (removing the ovaries, for instance), by manipulating their diets and by any number of genetic manipulations. The animals on which these indignities are inflicted do indeed become obese, not just functionally fat (like blue whales or hibernating ground squirrels). They tend to suffer from the same metabolic disturbances, including diabetes, that we do when we become obese.
It doesn’t matter, though, what technique is used to make the animals obese, they’ll still get that way or at least significantly fatter (just as Wade’s rats did) whether or not they can eat any more calories than otherwise identical animals that remain lean. They get obese not because they overeat, but because the surgery or breeding or genetic manipulation or even the change in diet disturbed the regulation of their fat tissue. They began stockpiling calories as fat, and then their bodies had to compensate: they ate more, if possible; they expended less energy if not. Often they do both.
Take, for example, the preferred method of making obese laboratory rodents from the 1930s through the 1970s. This was a surgical technique that required inserting a needle into a part of the brain known as the hypothalamus, which controls (not coincidentally) hormone secretion throughout the body. After the surgery, some of these rodents would eat voraciously and get obese; some would become sedentary and get obese; some would do both and get obese. The obvious conclusion, suggested first by the neuroanatomist Stephen Ranson (whose Northwestern University laboratory pioneered these experiments in the 1930s), is that the surgery has the direct effect of increasing body fat on these rodents. After the surgery, their fat tissue sucks up calories to make more fat; this leaves insufficient fuel for the rest of the body – what Ranson called “hidden semi-cellular starvation” – and “force[s] the body either to increase its general food intake or to cut down its expenditure, or both.”
The only way to prevent these animals from getting obese is to starve them – to inflict what a Johns Hopkins University physiologist in the 1940s called “severe and permanent” food restriction. If these animals are allowed to eat even moderate amounts of food, they end up obese. In other words, they get fat not by overeating, but by eating at all. Despite the fact that the surgery is in the brain, it has the effect of fundamentally altering the regulation of body fat, not appetite.
The same thing holds true for animals that are bred to be obese, for which obesity is in their genes. In the 1950s, Jean Mayer studied one such strain of obese mice in his Harvard laboratory. As he reported it, he could get their weights below that of lean mice if he starved them sufficiently, but they’d “still contain more fat than the normal ones, while their muscles have melted away.” Once again, eating too much wasn’t the problem; these mice, as Mayer wrote, “will make fat out of their food under the most unlikely circumstances, even when half starved.”
Then there are Zucker rats, which researchers began studying in the 1950s and are still a favorite obesity model today. Here’s a picture of a Zucker rat looking suitably corpulent:

These rats, like Mayer’s mice, are genetically predisposed to get fat. When these Zucker rats are put on a calorie-restricted diet from the moment they’re weaned from their mother’s milk, they don’t end up leaner than their littermates who are allowed to eat as much as they want. They end up fatter. They may weigh a little less, but they have just as much or even more body fat. Even if they want to be gluttons, which they assuredly do, they can’t, and still they get even fatter than they would have had they never been put on a diet. On the other hand, their muscles and organs, including their brains and kidneys, are smaller than they’d otherwise be. Just as the muscles in Mayer’s mice “melted away” when starved, the muscles and organs in these semi-starved Zucker rats are “significantly reduced” in size compared to those fat littermates who get to eat freely. “In order to develop this obese body composition in the face of calorie restriction,” wrote the researcher who reported this observation in 1981, “several developing organ systems in the obese rats [are] compromised.”
Let’s think about this for a second. If a baby rat that is genetically programmed to become obese is put it on a diet from the moment it’s weaned, so it can never eat as much as it would like – indeed, no more than a lean rat would eat -- it responds by compromising its organs and muscles to satisfy its genetic drive to grow fat. It’s not just using the energy it would normally expend in day-to-day activity to grow fat, it’s taking the materials and the energy it would normally dedicate to building its muscles, organs and even its brain and using that.
When these obese rodents are starved to death—an experiment that thankfully not too many researchers have done—a common result reported in the literature is that the animals die with much of their fat tissue intact. In fact, they’ll often die with more body fat than lean animals have when the lean ones are eating as much as they like. As animals starve, and the same is true of humans, they consume their muscles for fuel and that includes, eventually, their heart muscle. So as adults, these obese animals are also willing to compromise their organs and even their heart and their life to preserve their fat.
The message of eighty years of research on obese animals is simple and unconditional and worth restating: obesity does not come about because gluttony and sloth makes it so. Changing the regulation of the fat tissue makes lean animals obese, nothing else.
The amount of body fat on these animals is determined by a balance of all the various forces that work on the fat tissue – on the fat cells, as we’ll see -- to either put fat in or get fat out. Whatever’s been done to these animals to make them fat (surgery, genetic manipulation, etc.), the effect is literally to change this balance of forces so that the animals increase their fat stores. Now “eating too much” is a meaningless concept because virtually any amount of food is “too much.” The fat tissue is not reacting to how much these animals are eating but only to the forces making it accumulate fat. And because increasing body fat requires energy and nutrients that would otherwise be needed elsewhere in their bodies, they will eat more if they can. If they can’t – if they are on a strict diet – they will expend less energy because they have less to expend. They may even compromise their brains, muscles and other organs. Half-starve these animals and they’ll still find a way to stockpile calories as fat because that’s what their fat tissue is now programmed to do.
If this is true of humans, and there’s little reason to think it’s not, this is the explanation for the paradigm-challenging observation I mentioned earlier regarding extremely poor but overweight mothers with thin, stunted children. Both mother and children are half-starved. The children, thin and their growth stunted, respond as we’d expect. The mothers, however, have fat tissue that has developed its own agenda. (We’ll see shortly how this can happen.) It will accumulate excess fat, and does so, despite the fact that the mothers themselves, like their children, are barely getting enough food to survive. They must be expending less energy to compensate.
Before I leave the laws of adiposity and this animal research behind, I want to ask one more question: What do these laws and this research have to say about people who are habitually lean? Over the years, researchers have also created what we might call animal models of leanness – animals whose genes have been manipulated so they are leaner than they’d otherwise be. These animals will remain lean even when the researchers force them to consume more calories than they prefer – by infusing a tube into their guts, for instance, and pumping in calories directly. In such cases, the animals will surely have to increase their expenditure to burn off the calories.
The implication is as counterintuitive as anything we’ve discussed so far. Just as the animal research tells us that gluttony and sloth are side effects of a drive to accumulate body fat, it also says that eating in moderation and being physically active (literally, having the energy to exercise) are not evidence of moral rectitude. Rather they’re the metabolic benefits of a body that’s programmed to remain lean. If our fat tissue is regulated so that it will not store significant calories as fat, or our muscle tissue is regulated to take up more than its fair share of calories to use for fuel, then we’ll either eat less than those of us predisposed to be fat (the first case), or we’ll be more physically active (the second), or both because of it.
This implies that our emaciated marathoners are not lean because they train religiously and burn off thousands of calories doing so; rather they’re driven to expend those calories – and so perhaps to work out for hours a day and become obsessive long-distance runners -- because they’re wired to burn off calories and be lean. A greyhound will similarly be more physically active than a basset hound, not because of any conscious desire to exercise, but because its body partitions fuel to its lean tissue, rather than its fat.
It maybe easier to believe that we remain lean because we’re virtuous and we get fat because we’re not, but the evidence simply says otherwise. Virtue almost assuredly has no more to do with our weight than with our height. When we grow taller, it’s hormones and enzymes that spur our growth and we consume more calories than we expend as a result. Growth is the cause – increased appetite and decreased energy expenditure (gluttony and sloth) are the effects. When we grow fatter, the same is true as well.
We don’t get fat because we overeat; we overeat because we’re getting fat.
|
Gary Taubes is a Robert Wood Johnson Foundation Independent Investigator in Health Policy Research at the U.C. Berkeley School of Public health. He's the author of Why We Get Fat and What To Do About It (Knopf, 2011) and Good Calories, Bad Calories (Knopf, 2007). He studied applied physics at Harvard and holds a masters degree in engineering from Stanford and in journalism from Columbia University. A former staff reporter for Discover magazine, Taubes has written about medicine and nutrition for a wide variety of publications, including the New York Times Magazine. He's a contributing correspondent to the journal Science and the only print journalist to be a three-time winner of the National Association of Science Writers' science in society journalism award. |
Search Articles
Article Categories
Sort by Author
Sort by Issue & Date
Article Categories
Sort by Author
Sort by Issue & Date

